
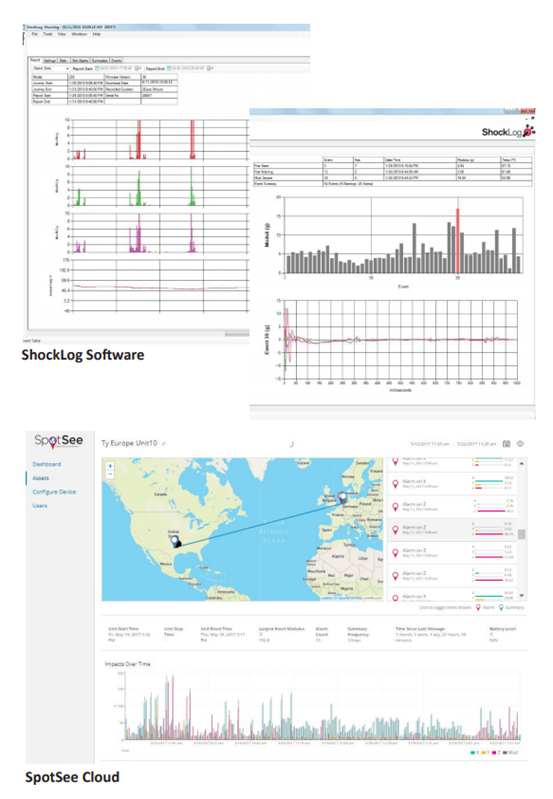
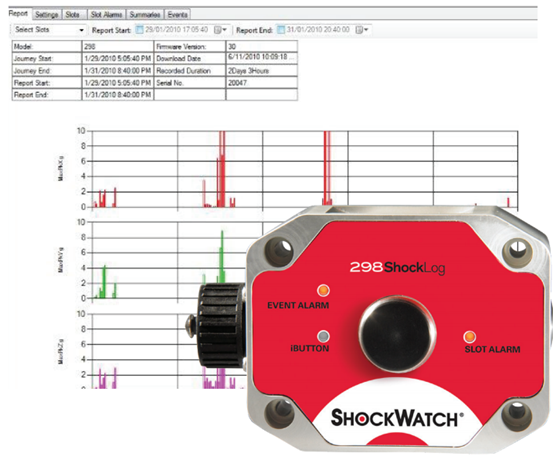
ShockLog? 298 monitors and records shock, vibration, and environmental conditions experienced by any type of structure or equipment, whether in use, in transit, or in storage. With the capacity to record data for 870 events and 262,000-time slots, the device alerts you whenever damage may have occurred so you can respond promptly.
Features:
l Records impact events; max peaks X, Y, and Z; gRMS; and internal temperature
l Sensors record direction, amplitude, and duration of impact force
l Field-proven triaxial piezoelectric accelerometer technology
l USB and iButton? data transfer options
l Self-contained unit design, free of cables and wires
l Programmable wake-up values for maximizing battery life
l User-definable warning and alarm levels
l LED lights for visual notification of alarms and warnings
l IP67-rated, RF-screened
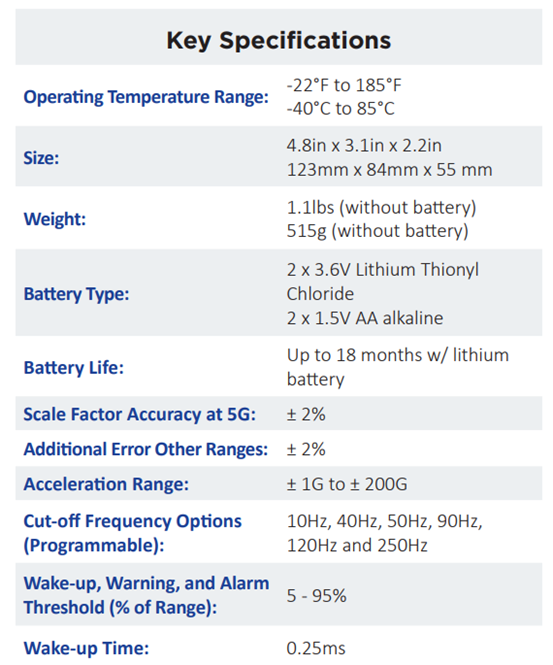
l Up to 18 months of battery life
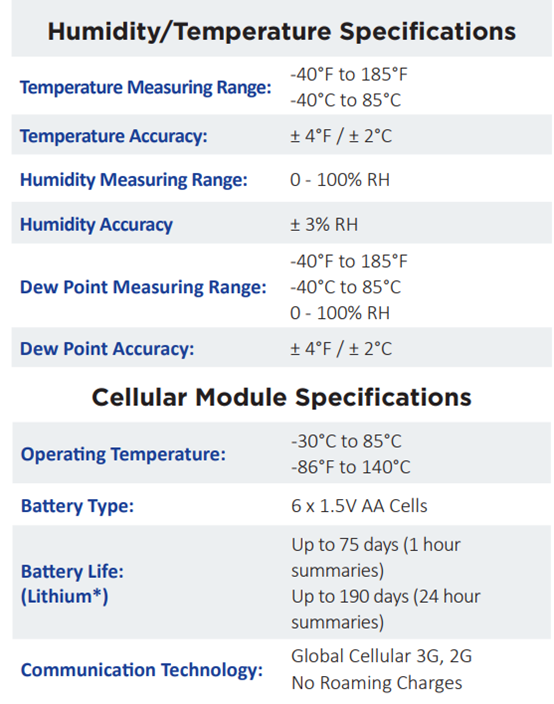
l Option to build temperature/humidity sensor into unit, or add a temperature/pressure/humidity accessory sensor
l Captures coordinates when event occurs at summary intervals with GPS (optional)
l GPS allows users, through hyperlinks, to pinpoint the exact location of an event and summary with the use of Google Maps (optional)
Benefits:
? Alert recipients and operators to inspect goods and equipment for potential damage
? Determine baseline damage boundaries
? Detect mishandling during shipping, operation and storage, enabling you to identify and assign accountability and take corrective action
? Make adjustments to product packaging, loading process, carriers, or mode of transport
? Help identify opportunities for improvement through journey profiling
Specifications:
SHOCKLOG 298 APPLICATIONS:
l Turbines
l Transformers
l Energy and Utilities
l Wind Energy
l Generators
l Medical and Scientific Equipment
l Large Medical Devices



